Solving complexity
“The expertise Leafcutter have solving complex, healthcare issues was very impressive and will progress forward with modern IT solutions in health care. It’s all about going from bench to bedside in science… if Leafcutter didn’t help us with creating a user friendly software then we will have nothing to go forward with.”
Dr Lisa Kouladjian O’Donnell
Overview
Project Need
A University of Sydney based medication management research team funded by the Cognitive Decline Partnership Centre has been working on a research project to optimise the quality use of medicines for people with cognitive and related functional decline.The academic researchers wanted to develop a new digital platform that incorporated research tools that have been developed and validated in various settings, to implement into practice.
They hypothesised that implementation of these research tools into practice will allow better medication management and quality use of medicines for people with cognitive and related functional decline. They needed a sophisticated online tool to assist pharmacists in managing the medications prescribed for their patients during their home and hospital consultations.
Project Goals
- To create a web-based software (CCDSS) that assists accredited pharmacists complete a medication review for people with dementia
- To capture de-identified medication/medical data (from software back-end) to analyse impact of the intervention
Technology
Laravel, Angular.js
Client
NHMRC Cognitive Decline Partnership Centre
What We Did
UI/UX Design, Responsive Web Design, Development
Our approach
From our initial conversation with the research team, we gave them the confidence that we can design and build a custom web application to bring their research to life. We developed a sophisticated web application that allows pharmacists to assist them in their medication recommendations and decision making for their patients. The system is web based, allowing it to be used in a range of scenarios encountered by the pharmacists. At the same time, the research team needed the web application to be scalable for future enhancements.
The platform
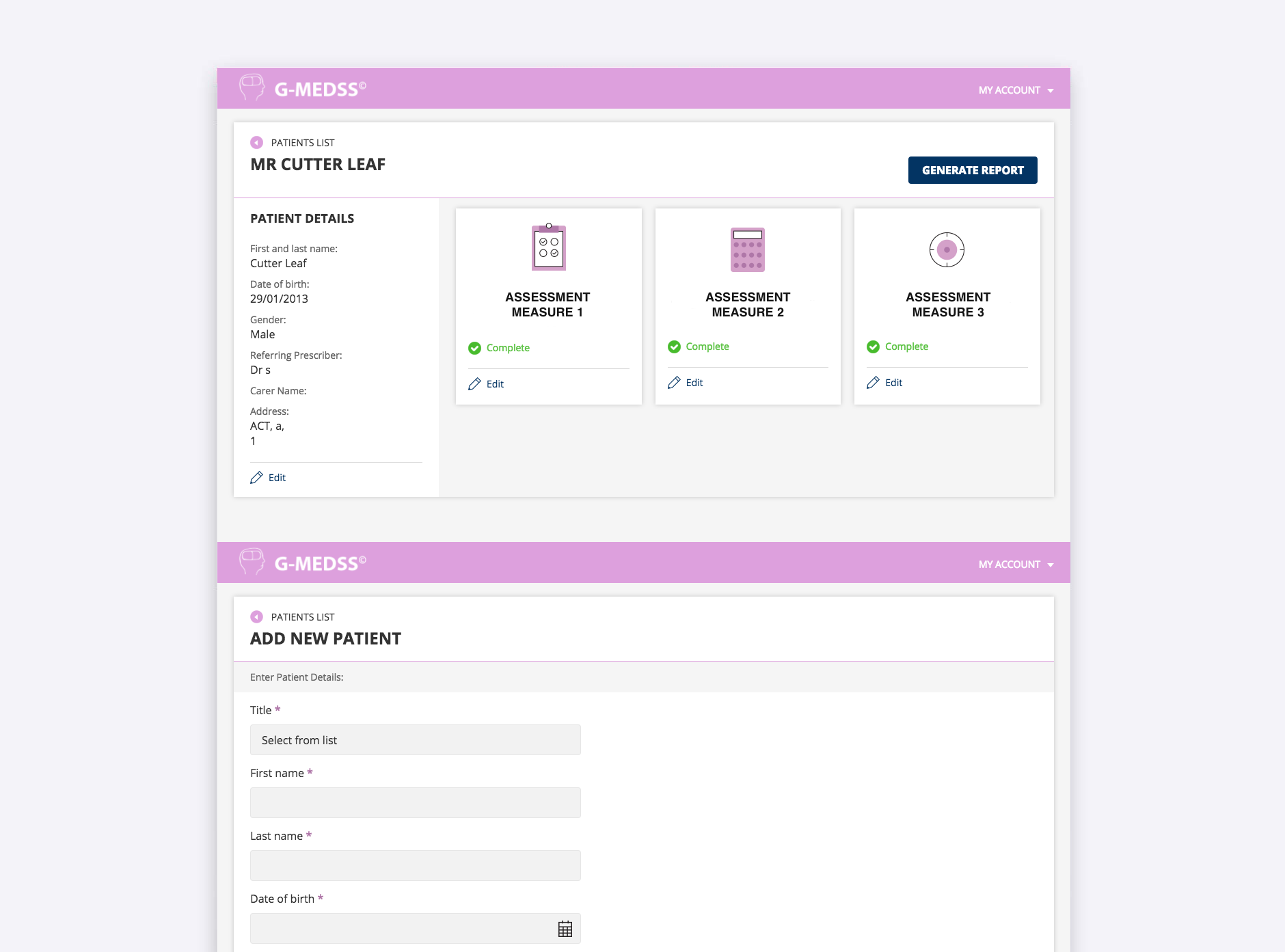
With the leadership of the research team, we designed and built the Goal directed Medication review Electronic Decision Support System © (G-MEDSS©) that combines various complex risk assessment measures. This combined system allows pharmacists to manage high risk medications for their patients, and guide the recommendations for quality use of medicines. This system is currently being tested in various clinical settings.
Our audience are specially trained pharmacists (accredited pharmacists). G-MEDSS© was designed and built to complement usual work flow with simplicity and sophistication in mind. G-MEDSS© is responsive, has offline capability and functions that are user intuitive.
Key features of G-MEDSS include:
- Responsive web app with optimal user experience across all devices and all screen resolutions
- Various complex risk assessment measures
- Secured login access with admin functionality & multi user logins
- Offline mode to allow users to access remotely during home visits
- Ability to de-identified medication/medical data from the backend for data analysis
- Generates customised reports in PDF format to allow the output of reports with different fields and information to suit users
- Tracking setup and implementation of Google Tag Manager and Google Analytics – for continual monitoring and refinement of the user experience.
Results
The creation of G-MEDSS© allows for pharmacists participating in current research projects to make decisions based on quantitative results and communicate them clearly to the referring general practitioner and the consumer.
More exciting times lies ahead for the research team as they test G-MEDSS© in hospitals, the community and residential aged care settings, and extrapolate useful analytics through this tool which, if proven to be effective and useful, can be used as an intervention beyond the clinical trial setting.